 Image copyright
Image copyright
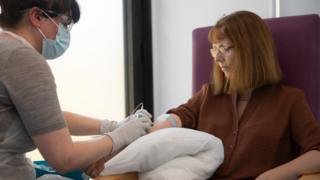
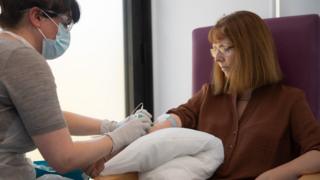
University of Oxford/PA Media
There are now 23 coronavirus vaccines in clinical trials around the world – including at Oxford University
The UK government has signed deals for 90 million doses of promising coronavirus vaccines that are being developed.
The vaccines are being researched by an alliance between the pharmaceutical companies BioNtech and Pfizer as well as the firm Valneva.
The new deal is on top of 100 million doses of the Oxford University vaccine being developed by AstraZeneca.
However, it is still uncertain which of the experimental vaccines may work.
A vaccine is widely seen as the best chance of getting our lives back to normal.
Research is taking place at an unprecedented scale – the world became aware of coronavirus at the beginning of the year, but already more than 20 vaccines are in clinical trials.
Some can provoke an immune response, but none has yet been proven to protect against infection.
The UK government has now secured access to vaccines that use three completely different approaches:
- 100m doses of the Oxford vaccine made from a genetically engineered virus
- 30 million doses of the BioNtech/Pfizer vaccine, which injects part of the coronavirus’ genetic code
- 60 million doses of the Valneva, which uses an inactive version of the coronavirus
Using different styles of vaccine maximises the chance that one of them will work.
Kate Bingham, the chair of the government’s Vaccine Taskforce, said: “The fact that we have so many promising candidates already shows the unprecedented pace at which we are moving.
“But I urge against being complacent or over optimistic.
“The fact remains we may never get a vaccine and if we do get one, we have to be prepared that it may not be a vaccine which prevents getting the virus, but rather one that reduces symptoms.”
If an effective vaccine is developed then health and social care workers, as well as those at highest risk of the disease, will be prioritised.
It is possible a vaccine will be proven effective by the end of 2020, but wide-scale vaccination is still not expected until next year.
The education secretary, Gavin Williamson, told BBC Breakfast that vaccine development was “an incredibly long process and we are doing it at breakneck speed” but that we should expect a Covid 19 vaccine “after winter”.
Media playback is unsupported on your device
The announcement also includes an agreement with AstraZeneca to buy treatments made from neutralizing antibodies, which can disable the virus.
These could be given to people who cannot be vaccinated because they have a weakened immune system or are having treatment for cancer.
Meanwhile, the government is hoping to get half a million people to sign up to trials of vaccines in the UK through the NHS Covid-19 vaccine research registry website.
At least eight large scale coronavirus vaccine trials are expected to take place in the UK.
Prof Chris Whitty, Chief Medical Officer, said: “Now that there are several promising vaccines on the horizon, we need to call again on the generosity of the public to help find out which potential vaccines are the most effective.”
Follow James on Twitter
- INSIDE HEALTH: Why is there an elevated risk from Covid-19 for some groups in society?
- DIET AND MENTAL WELLBEING: Does the food you eat help or hinder you emotionally…


